
Regulatory Services
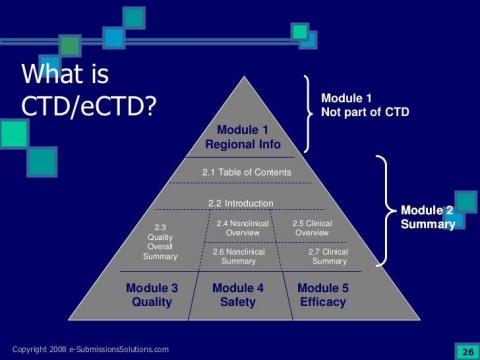
- CTD files preparation and review.
- Response to deficiency letters.
- Monitoring and auditing bioequivalence studies.
- File registration and follow up.
- GMP consultation (site layout, documentation system training.
- Patent check and “free to operate opinion” in KSA.
Sana Pharma offers group of regulatory services such as:
Sana Pharma also provides
- Technical opinion concerning the registrability of products or manufacturing sites through the review of the product dossiers, Drug Master Files, Site Master Files.
- Expert reports for clinical and none clinical studies and summaries (CTD modulesII, IV & V).
- Periodic safety update reports (PSUR).
- Our qualified experts are ready to work as independent party for inspection of API’s or pharmaceutical manufacturing sites for regulatory purposes.



